CD Marker
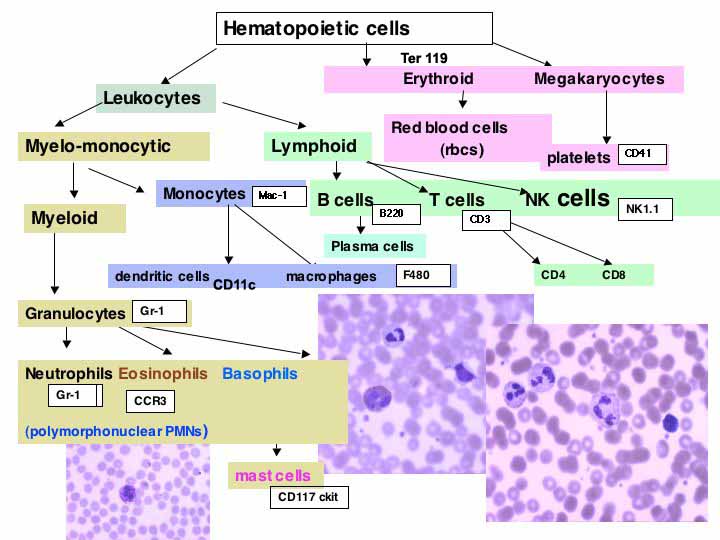
(full size chart)
Aim: to determine the distribution and pattern of CD markers in mouse spleen from wild type or test mouse, using enzyme labels for BRIGHTFIELD MICROSCOPY
Material:
-
Frozen sections air dried and used, after 30 minutes or the next day or stored at minus 80 degrees for use in 2 weeks
-
10% goat serum, 1%BSA/PBA, blocking buffer
-
PBS, washing buffer
Positive control and :
Negative control: frozen sections of wild type mouse spleen: one slide for no primary antibody and one slide for each CD marker
Antibodies:
1. Biotinylated CD3e (Tcells) BDPharmingen Cat. No. 553059 –-use at 2.5 ug/ml --1:200
2. Biotinylated CD4(L3T4/(RM4-5) ; BD Pharmingen Cat. No. 553045 at 1 ug/ml; -1: 500
3. Biotinylated CD8a (Ly-2) ; BD Pharmingen Cat. No. 553029 use at 2.5 ug/ml;-----1:200
4. Biotinylated CD45/B220( B cells RA3-6B2); BDPharmingen Cat. No. 553086 at 2.5ug/ml--1:200
5. Biotinylated CD11b/Mac-1 (monocytes and activated neutrophilsM1/70); BD Pharmingen Cat. No.553309 at 1ug/ml—1:500
6. Biotinylated F480 (resident macrophages); BioSource International Cat. No.AMU 0089 use at--1:50.
And Serotec Cat. No. MCA497B
7. Biotinylated Gr-1(neutrophils/ granulocytes Ly-6G) ; BD Pharmingen Cat. No. 553125 use at----1:200
8. Rabbit anti AsialoGM1 for NK cells; WAKO Cat. No. 986-10001 use at --1:4000
9. Positive control: Biotinylated rat anti mouse CD 31 ) use at ----1:500
BD Pharmingen Cat. No. 09331A (Cat No. 01951D also works well )
10. Negative control: slide receives buffer alone, followed by secondary antibody
Procedure:
-
Air dry frozen sections for 30 minutes
-
Fix in acetone (Fisher Cat. No. A16-4) for 10 minutes,
-
Wash in PBS, 3 changes
-
Remove endogenous peroxidases by immersing in 0.03% H2O2 for 30 minutes
-
Wash in PBS, 3 changes
-
??????Set up on autostainer
-
Overlay tissue sections with 1%BSA/ PBS
-
Remove endogenous biotin by incubating first with 0.1% avidinin PBS for 15 minutes,
-
followed by 3 PBS washes
-
then by incubating with 0.01% biotin in PBS for 15 minutes
-
followed by 3 PBS washes
-
Overlay with antibodies diluted in 1% BSA/PBS
-
Incubate for 30 minutes at room temperature
-
Wash 3 times in PBS
- IF any slide received NON-biotinylated anti CD antibodies, the specific binding has to be detected using a Biotinylated Goat anti-Rat 1:500 (BectonDickinsonPharmingen Cat No.554-014)
FOR WAKO’s anti Asialo GM1, use HRP anti Rabbit secondary at 1: 100
-
Incubate primary antibodies for 30 minutes at room temperature
-
Wash 3 times in PBS
-
Incubate with HRP Streptavidin 1:500 (Jackson Immunoresearch Cat No.016-030-084)
-
Wash 3 times with PBS
-
Overlay with VIP for 2-6 minutes (purple substrate--Vector labs Cat No. SK 4600) or the usual AEC substrate--red substrate.
-
Wash 3 times with PBS
- Counterstain nuclei with Mayer’s hematoxylin
-
Wash 3 times with PBS
-
Coverslip using Aquamount (Fisher Cat. No BM-01) Or 50% glycerol/PBS)
The positive control tissue section with anti CD31 should demonstrate blood vessels only, as positive staining control.
The negative tissue control should only show counter-stained nuclei
| 