So you want to do an immunohistochemistry assay?
If the antibody has not been tested on tissues before, it will take quite a bit of effort to
determine if the antibody is specific--- the assays will thus need to be optimized.
There should definitely be a plan BEFORE you start throwing antibodies on tissue sections. Initial questions that need to be answered:
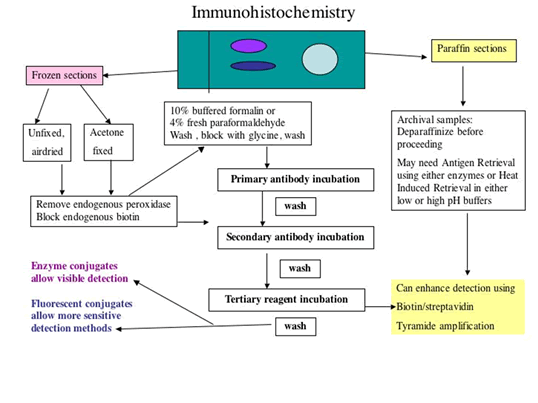
1. Are the frozen sections going to be used fixed or not and if fixed what fixative?
2. what are the negative and positive control reagents?
3. what are the expected negative and positive controls on tissue sections?
4. what secondary reagents are you planning to use?
5. and thus what are the blocking reagents required?
6. are overnight incubations needed?
7.will a fluorescence OR enzyme detection system be used?
You will find some hints on the powerpoint presentations on the other histology core website:http://cancer.ucsd.edu/histology/
Other Questions:
1.Are you going to immunostain frozen sections or paraffin sections?
2. Is your antibody a polyclonal rabbit or polyclonal goat or mouse monoclonal or rat
monoclonal?
3, What is the tissue you are testing, rabbit tissue or rat tissue or mouse tissue?
4. Is the antibody sold as being tested and shown to work well in immunohistochemistry assays?
5. What is the expected Positive control tissue for the antibody?
6. What is the expected Negative control tissue for the antibody?
7. Do you have cells in tissue culture that you know are positive or negative for the antibody that
can be used as controls in the assay?
8. What is the concentration of antibody that you used on Westerns to obtain the positive
staining?
Here are some suggestions as to how to proceed with an immunohistology assay
A. CONTROLS:
-
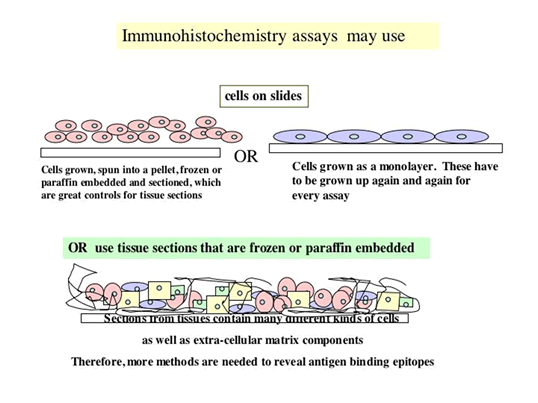
Positive control: Cells or tissue expected to be positive. Positive controls may also be the cells that are known to express the epitope, that are grown in tissue culture up to about 100 million, taken and made into a pellet, frozen in OCT compound, following the tissue freezing protocol, and sectioned.
-
Negative control: Cells or tissue expected to be negative.
-
Blocking peptide (see below)
Reagent controls:
-
1% BSA/PBS (Bovine Serum Albumin in Phosphate Buffered Saline) could serve as a reagent negative control for secondary reagents
-
Rabbit IgG Dako Cat.# 1505 Negative control reagent at 1-5 ug/ml
-
Rabbit anti vWf Dako Cat# 0936 (pre-diluted). To be diluted for use of frozen sections, and is a positive for blood vessels which are present in all tissue.
-
Mouse anti Vimentin Dako Catalog# N1521 prediluted, ready to use, as a technical positive control when using mouse antibodies as test
-
Mouse IgG at 1-5 ug/ml
-
Further controls included staining of cells expressing higher levels of epitope in question, and using the immunizing peptide to compete out the reactivity of the antibody, resulting in a negative stain.
B. FIX OR NOT to FIX? IMMUNOSTAIN PLAN:
Plan to run 3 initial sets of slides: Set 1 sections will be used unfixed, airdired;
Set 2 sections will be used after acetone fixation;
Set 3 sections will be used for fixation using 4% paraformaldehyde (or with 10% buffered formalin)
It is important to note that Set 3 sections, the FORMALIN/PARAFORMALDEHYDE SET should only be fixed AFTER removing endogenous peroxidases (if using HRP labeled detection), AND after blocking endogenous biotin (if using biotin in the system)
WASH BUFFERS:
Phosphate Buffered Saline (PBS) with a pH 7.4 may be the best buffer to use when testing new reagents.
TBST= Tris buffered saline with 0.05% Tween 20; The tween in the buffer TBST helps to disperse the reagent evenly over entire section, and may result in more even staining for an inexperienced user.
C. Block ENDOGENOUS PEROXIDASES
If the plan includes fixation of the frozen sections in formalin containing fixative, it is very important that blocking of endogenous peroxidases, and blocking of endogenous Biotin is performed BEFORE the fixation in formalin. For removal of endogenous peroxidases that are present in blood cells, in blood vessels of all tissues: Immerse slides in slide holder in 0.03% H2O2 in PBS for 30 minutes at room temperature, followed with 3 buffer washes
D. Block ENDOGENOUS BIOTIN
-
IF the protocol USES BIOTIN in the LINK or in the LABEL,
Overlay with 0.1% avidin for 10 – 15 minutes (following manufacturer’s suggestions), followed by 3 buffer washes
-
This step is followed by overlaying the washed sections with 0.01% biotin for 10 – 15 minutes (following manufacturer’s suggestions), followed by 3 buffer washes
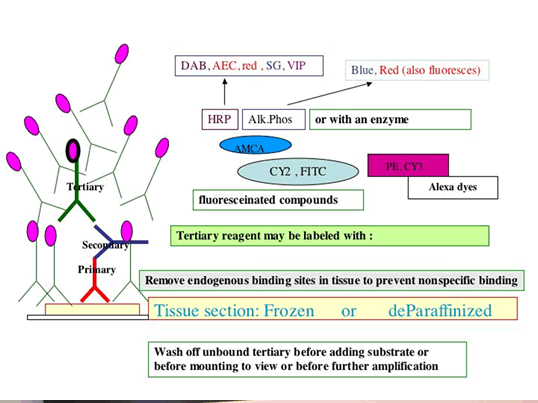
E. IMMUNOSTAINING:
-
Overlay sections with blocking solution to block non-specific protein binding.
Do not let the sections dry out at any time or this will contribute to high background. Incubate in humid chamber to help prevent this
-
Overlay sections sequentially with Reagents 1 (Primary antibody used at predetermined dilution) or negative reagent controls) and incubate in humidified chamber for the specified length of time.
Wash in wash buffer and overlay with Secondary Reagents at predetermined dilutions, followed by washes, and then use Substrate, FRESHLY made, following manufacturer's directions precisely ( no need for substrate of course if Fluorescent tagged detection is used).
A brief immersion in nuclear counterstain, followed by buffer washes then precedes coverslipping in aqueous mounting media for viewing and photography.
MATERIALS:
-
BSA—Sigma Cat. No. A2153
Green staining dish for organic solvents (VWR Cat.# 25608-904)
-
White staining dish VWR Cat.# 25608-906
-
Slide holder (VWR Cat# 25608-868)
-
PBS buffer phosphate buffered saline pH 7.4
-
TBS buffer : a. Biogenex Cat. # HK098-5K
-
or DAKO Cat.# S1968
-
Tween 20 (Polyoxyethylene-Sorbitan Monolaureate)—Sigma Cat. No. P1379
-
Avidin/Biotin Blocking Kits: a. Vector Cat. No. SP-2001
-
or from Dako Cat. No. X0590
-
or make from Sigma Avidin Cat no A 9275
-
or make from Sigma Biotin Cat no B4501
-
Mouse Anti Human Vimentin—DAKO Cat. No. N1521 prediluted
-
Mouse IgG monoclonal supernatant from P3X63 Ag8, usually at 5 ug/ml, negative reagent control
-
Rabbit anti human vWf Dako Cat # N1505
-
Rabbit IgG Dako Cat.# X0936 negative rabbit reagent control, use at 5 ug/ml
-
AEC substrate: a. Vector Cat. No. SK-4200
-
or Biogenex Cat # HK092-5K
-
30% H2O2 to be diluted for use —Fisher Cat. No. H325-100
-
Aquamount—Fisher Cat. No. BM-01
-
Mayer’s Hematoxylin— Sigma Cat. No. MH532-1L