HEMATOLOGY
UCSD Murine Hematology and Coagulation Core Laboratory
Dr. Dzung T. Le, M.D., Ph.D., Director
To arrange for hematology, serum chemistry, and coagulation services, contact the lab via email to set an appointment and receive instructions for the shipping and handling of samples.
To schedule services: Qiongyu Chen at q2chen@ucsd.edu
Lab phone: (858) 534-3172
Lab: BRF II, 4217 LL
Service prices:
| Service |
Price per sample |
Includes |
Required Sample |
| HEMATOLOGY |
| · Complete blood count |
$30 |
CBC, WBC differential, Platelet count; Smear for staining and viewing upon request. |
Whole Blood - 100 uL in Microtainer EDTA Tube (Lavender, P/N: BD 365974) |
| CHEMISTRY |
| ·Comprehensive Metabolic Panel |
Collaboration Only |
Albumin, ALP, ALT, AST, Anion Gap, Bicarbonate, BUN, Calcium, Creatinine, Chloride, Glucose, Potassium, Sodium, Total Bilirubin, and Total Protein |
Serum - 300uL per panel |
| · Lipid Panel |
Cholesterol, Triglycerides, HDL-cholesterol, LDL-cholesterol (calculated) |
| COAGULATION |
| · PT |
$60 |
Prothrombin time |
Citrated Plasma
Volume required dependent on assays requested |
| · aPTT |
$60 |
Activated partial thromboplastin time |
· Coagulation Factor: II, V,
VII, VIII, IX, X, XI, and XII |
$60 |
Coagulation factor activity
(each tested and priced individually)
|
| · Fibrinogen |
$60 |
Fibrinogen activity |
| · Antithrombin |
$60 |
Antithrombin activity |
| · Protein C |
$60 |
Protein C activity |
| · Protein S |
$60 |
Protein S activity |
| · Plasminogen |
$60 |
Plasminogen activity |
| · Antiplasmin |
$60 |
Antiplasmin activity |
| · von Willebrand Factor |
$60 |
vWF activity |
Additional Services: Platelet aggregometry are also available (collaboration only). Inquire for prices and methods.
HEMATOLOGY special consideration: This service is only available locally (San Diego County), as samples are only viable 4 hours after collection. Overnight shipping of samples will not be accepted.
CHEMISTRY special considerations:
- Volume required for one panel = 300ul serum
- (i.e., Metabolic & Lipid panel requested, 300ul + 300ul = 600ul required)
- To meet the required volume for analysis, the researcher has several options:
- Pooling samples
- Same mouse, multiple survival bleeds: may be taken over regularly spaced intervals to allow the mouse to recover. Serum is prepared after each bleed, stored, and later pooled once sufficient.
- Same mouse group: matched for age, gender, genotype, and treatment conditions.
- Termination bleed
- May produce sufficient serum volume for one panel
- Dilution of sample with deionized water
- It is not recommended to dilute the sample more than 1:2 (100uL DI water to 200uL serum). If over-diluted, levels of interest (e.g. LDLc, Chloride) may not be present at high enough levels to reach the threshold of detection and will produce inaccurate or no results.
- Fasting - plan accordingly for time of food intake or restriction, prior to sampling, as this may affect results.
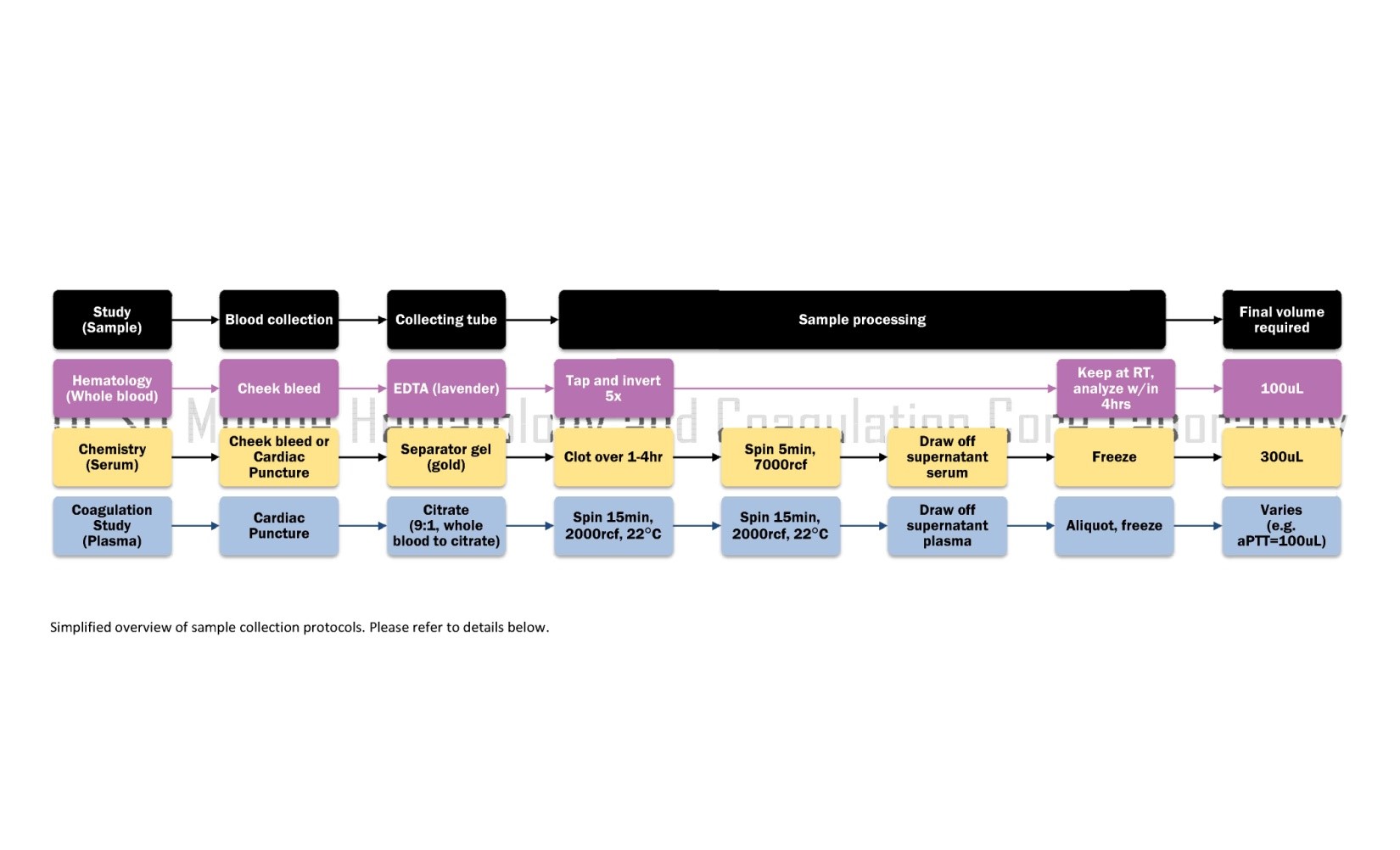
Click image to enlarge
BLOOD COLLECTION PROCEDURES
Submandibular Vein or Artery Blood Collection (for HEMATOLOGY or CHEMISTRY)
- The confluence of the submandibular vein, orbital vein, and other facial veins is accessible at the cheek
- Mice are kept awake but must be restrained effectively
- The mouse is lifted into the air by the scruff, allowed to hang into its most relaxed position, then the vascular bed is punctured at the back of the jaw with a lancet or 20ga. needle
- For HEMATOLOGY: Blood drops are collected into a Microtainer EDTA Tube (Lavender, P/N: BD 365974) and immediately mixed by tapping and inverting the tube five times to ensure anticoagulation
- For CHEMISTRY: Blood drops are collected into Microtainer Serum Separator Tube(s) (SST, Gold, P/N: BD 365967)...see "Processing" below for next steps
- Light pressure is applied to the cheek with a Kimwipe tissue to close the puncture and stop bleeding
- Mice are returned to their cage
- CBC samples are kept at room temperature and must be tested within 4 hours of collection
Cardiac Puncture Blood Collection (for CHEMISTRY or COAGULATION STUDIES)
Materials
| For Serum CHEMISTRY |
For Citrated Plasma COAGULATION STUDIES |
| 1. Inhalant anesthetic |
1. Inhalant anesthetic |
| 2. 1mL plastic syringe with a 25ga. needle |
2. 1mL plastic syringe with a 25ga. needle
loaded with 30 uL of Buffered citrate (0.06 mole/L sodium citrate, 0.04 mole/L citric acid, pH 7.4)
|
| 3. Microtainer Serum Separator Tube(s)
(e.g., SST, Gold, P/N: BD 365967)
|
3. Microtubes containing sufficient additional citrate to achieve a final ratio of nine parts whole blood to one part citrate (9:1) |
Procedure
- Mice are kept under general (inhalant) anesthesia
- One of two methods may be used
- Open - body cavity is opened to expose the heart
- Closed - a needle is inserted through the intact skin, between the ribs, to reach the heart
- Needle is inserted to the heart and up to 1mL of blood is withdrawn. Note the volume.
- Blood is emptied into the microtube
- For CHEMISTRY: Allow blood to stand and clot at room temperature up to 4 hours...see "Processing" below for next steps
- For COAGULATION STUDIES: Blood is immediately mixed well by tapping and inverting the tube five times to ensure proper anticoagulation. (If the sample is not mixed well, blood will clot and cannot be analyzed) ...see "Processing" below for next steps
- Mice are euthanized by cervical dislocation
PROCESSING SAMPLES
Processing Samples for HEMATOLOGY (CBC)
- Whole blood samples should be collected into tubes with an anticoagulant, preferably EDTA.
- Samples must be tested within 4 hours of collection to avoid coagulation and to ensure accurate results.
- Analysis is done using a Hemavet 950FS Multi-Species Hematology System (Drew Scientific, CT) programmed with species-specific (e.g., mouse, monkey, dog) settings.
- Background checks are run before sample analysis to verify that background counts are within acceptable limits.
- A mouse control reference is supplied by the manufacturer and is tested each time samples are run for calibration control.
- Control reference for other species are not kept in stock; contact our lab to discuss ordering.
- All samples are tested in duplicate (unless otherwise specified) for a Complete Blood Count (CBC) with leukocyte differential, and a Platelet count.
- Results may be given as individual sample report sheets and/or a cumulative excel file.
- An unstained whole blood smear is prepared for each sample and is available for reference upon request.
Processing Samples for CHEMISTRY (Comprehensive Metabolic Panel, or Lipid Panel)
- Collect blood into a Microtainer Serum Separator Tube(s) (SST, Gold, P/N: BD 365967).
- Blood is allowed to clot over 4 hours at room temperature.
- Blood in the SST is then spun for 5 minutes at 7000 rcf.
- Serum supernatant is removed and placed into a 1.5mL microtube.
- If not immediately analyzed, serum is aliquoted and frozen at -80°C. Avoid multiple freeze-thaw cycles.
- Analysis is done using a Cobas 8000 automated chemistry analyzer (Roche) with a general coefficient of variance of <5%.
Processing Samples for Coagulation Studies (e.g. PT, aPTT, Coagulation Factors)
- Collect blood into a microtube containing sufficient additional citrate to achieve a final ratio of nine parts whole blood to one part citrate (9:1).
- Sample is centrifuged twice at 2000 rcf for 15 min at 22°C.
- Plasma is drawn off the top, aliquoted, and frozen at -80°C within two hours of puncture. Avoid multiple freeze-thaw cycles.
- Analysis varies by assay, please see details below.
Materials for All Clotting Time Studies*
- ST4 semi-automated mechanical coagulation instrument (Diagnostica Stago, NJ)
- 4-well cuvettes
- Magnetic mixing ball
- Citrated plasma samples
Prothrombin Time (PT)
Measures the time to clot formation, indicating the activity of the extrinsic and common coagulation pathways and their involved clotting factors.
Additional Materials*
- Thromboplastin reagent
Procedure
- Instrument, cuvettes, mixing balls, and thromboplastin reagent are pre-warmed to 37℃
- 30uL of citrated plasma samples, in duplicate, are incubated within cuvette wells at 37℃ for 3 minutes
- 60uL of thromboplastin reagent is added to each well to initiate clotting
- Time until clot formation is measured in seconds
Activated Partial Thromboplastin Time (aPTT)
Measures the time to clot formation, indicating the activity of the intrinsic and common coagulation pathways and their involved clotting factors.
Additional Materials*
- APTT reagent
- 25mM CaCl2
Procedure
- Instrument, cuvettes, mixing balls, and CaCl2 are pre-warmed to 37℃
- 30uL of citrated plasma samples, in duplicate, are added to each well, followed by 30uL of aPTT reagent, and then incubated at 37℃ for 5 minutes
- 30uL of CaCl2 is added to each well to initiate clotting
- Time until clot formation is measured in seconds
Factor VIII
Key coagulation factor of the intrinsic pathway; activity levels are based on correction of clotting time for plasma deficient of the factor of interest and is reported as a percent.
Additional Materials*
- HN/BSA
- aPTT reagent
- 25mM CaCl2
- Citrated plasma deficient of factor VIII‡
- Normal mouse plasma (NMP) BL/6 pool
Procedure
- Instrument, cuvettes, and mixing balls are pre-warmed to 37℃
- Citrated plasma samples are diluted 1/20 in HN/BSA
- 30uL of sample dilutions, in duplicate, are added to each well, followed by 30uL of aPTT reagent, and then 30uL of citrated plasma deficient of factor VIII‡, and incubated at 37℃ for 5 minutes
- 30uL of CaCl2 is added to each well to initiate clotting
- Time until clot formation is measured
- Time is interpolated on a standard curve based on NMP serial dilutions and reported as %BL/6
Factor IX, Factor XI, and Factor XII
Follow the factor VIII method, using plasma deficient of the specific factor being measured in place of factor VIII‡ deficient plasma.
Factor VII
Key coagulation factor of the extrinsic and common pathway; activity levels are based on correction of clotting time for plasma deficient of the factor of interest and is reported as a percent.
Additional Materials*
- Owren's Veronal Buffer
- Thromboplastin reagent
- Citrated plasma deficient of factor VII§
- Normal mouse plasma (NMP) BL/6 pool
Procedure
- Instrument, cuvettes, and mixing balls are pre-warmed to 37℃
- Citrated plasma samples are diluted 1/200 in Owren's Veronal Buffer
- 30uL of sample dilutions, in duplicate, are added to each well, and then 30uL of citrated plasma deficient of factor VII§, and incubated at 37℃ for 3 minutes
- 60uL of thromboplastin reagent is added to each well to initiate clotting
- Time until clot formation is measured
- Time is interpolated on a standard curve based on NMP serial dilutions and reported as %BL/6
Factor II, Factor V, and Factor X
Follow factor VII method, using plasma deficient of the specific factor being measured in place of factor VII§ deficient plasma.
Fibrinogen Activity Assay
Activity of fibrinogen, activated by thrombin, is measured in time to clot formation.
Additional Materials*
- Owren's Veronal Buffer
- Bovine thrombin reagent
- Fibrinogen reference plasma
Procedure
- Citrated plasma samples are diluted 1:10 in Owren's Veronal Buffer
- 45uL of sample dilutions, in duplicate, are incubated at 37℃ for 3min
- 22.5uL of bovine thrombin reagent are added to activate fibrin clot formation
- Standard curve generated by dilutions of a standardized plasma of calibrated fibrinogen concentration
- Measured clotting times are converted to fibrinogen concentration using the standard curve
Materials for all Immunologic and Chromogenic Assays✝
- Versa Max microtiter plate reader (Molecular Devices, CA)
- 96-well microtiter plate
- Citrated plasma samples
- Normal mouse plasma (NMP)
AntiThrombin Assay
Antithrombin activity is measured by level of inhibition of Factor Xa.
Additional Materials✝
- HN/BSA
- Factor Xa/Heparin reagent
- 1.25 mg/ml chromogenic substrate S-2765
Procedure
- Plasma samples may be diluted to 1:40 - 1:200 in HN/BSA
- Standard curve is prepared with each plate by diluting NMP 1:20 to 1:640 in HN/BSA, analyzed simultaneously on the sample plate
- Plate wells are loaded as follows:
| Sample dilutions, in duplicate |
40uL |
37℃ |
3min |
|
| Factor Xa/Heparin reagent |
40uL |
|
|
|
| Chromogenic substrate |
40uL |
|
|
Color development |
- Plate is read at 405nm
- Absorbance are converted to %NMP Antithrombin using the standard curve
Protein C Assay
Measured by the level of chromogenic substrate cleaved by activated protein C.
Additional Materials✝
- TBS with 100mM CsCl
- 2 u/mL Protein C activator
- 2.5mM chromogenic substrate specific for activated Protein C (APC)
- 20% acetic acid
Procedure
- Plasma samples may be diluted to 1:10 - 1:20 in TBS-CsCl
- Plate wells are loaded as follows:
| Sample dilutions, in duplicate |
10uL |
37℃ |
15min |
|
| Protein C activator |
25uL |
37℃ |
15min |
|
| Chromogenic substrate |
25uL |
37℃ |
1hr |
Color development; plate must be covered |
| Acetic acid |
25uL |
37℃ |
1hr |
Stop reaction |
- Plate is read at 405nm
- Standard curve is prepared by diluting NMP 1:4 to 1:64 in TBS, analyzed simultaneously on the sample plate
- Absorbance are converted to %NMP protein C using the standard curve
Protein S Assay
Protein S antigen is detected by binding to an antibody, then secondary binding by a conjugated antibody that will produce a detectable color change with the addition of a substrate.
Additional Materials✝
- 10 ug/uL rabbit anti-human protein S polyclonal antibody prepared in 50mM Na2CO3, pH 9.6
- TBS
- 3% BSA in TBS
- 1% BSA in TBS
- 0.05% Tween 20 in TBS
- Horseradish peroxidase (HRP)-conjugated rabbit anti-human Protein S polyclonal antibodies diluted 1/1000 in 1%BSA in TBS
- TMB peroxidase substrate
- 1N H2SO4
Procedure
- Plasma samples may be diluted to 1:100 - 1:200 in TBS/1%BSA
- Plate wells are loaded as follows:
| Rabbit anti-human protein S Ab |
100uL |
5℃ |
Overnight |
|
| 3%BSA in TBS |
200uL |
37℃ or 5℃ |
3-5hr or Overnight |
Block |
| 1%BSA in TBS |
|
|
|
Wash 1x |
| Sample dilutions, in duplicate |
100uL |
5℃ |
Overnight |
|
| 0.05% Tween 20 in TBS |
|
|
|
Wash 5x |
| Diluted HRP-conjugated rabbit Ab in TBS/1%BSA |
100uL |
5℃ |
Overnight |
|
| 0.05% Tween 20 in TBS |
|
|
|
Wash 5x |
| TMB peroxidase substrate |
|
|
4hr |
Color development |
| H2SO4 |
100uL |
|
|
Stop reaction |
- Plate is read at 655nm during development then at 450nm after reaction is stopped
- Log-log standard curve is prepared by diluting NMP 1:25 to 1:800 in TBS/1%BSA, analyzed simultaneously on the sample plate
- Absorbance are converted to %NMP Protein S using the standard curve
Plasminogen Activity Assay
Activity is measured by the level of chromogenic substrate cleaved by plasmin or urokinase-activated plasminogen.
Additional Materials✝
- 100mM Tris, pH 8.5 with 8.3mM EACA (Tris/EACA)
- HN/BSA
- 2500 Ploug U/mL urokinase
- 1.2mM chromogenic substrate specific for plasmin and urokinase-activated plasminogen in HN buffer
- 20% acetic acid
Procedure
- Plasma samples are diluted to 1:50 in Tris/EACA
- Plate wells are loaded as follows:
| Sample dilutions, in duplicate |
60uL |
37℃ |
90sec |
|
| Urokinase |
20uL |
|
60sec |
|
| Chromogenic substrate in HN |
100uL |
|
10min |
Color development |
| Acetic acid |
100uL |
|
|
Stop reaction |
- 100uL of is added to each well for color development
- Plate is read at 405nm
- Log-log standard curve is prepared by diluting normal mouse plasma (NMP) 1:10 to 1:320 in Tris/EACA, analyzed simultaneously on the sample plate
- Absorbance are converted to %NMP plasminogen using the standard curve
Alpha-2 AntiPlasmin Assay
Antiplasmin activity is measured by level of inhibition of plasmin.
Additional Materials✝
- TBS with 120mM methylamine chloride
- 10ug/mL plasmin
- 1.5mM chromogenic substrate specific for plasmin
- 20% acetic acid
Procedure
- Plasma samples are diluted to 1:50 in TBS with methylamine chloride
- Standard curve is prepared by diluting NMP 1:10 up to 1:1280 in TBS with methylamine chloride
- Plate wells are loaded as follows:
| Sample dilutions, in duplicate |
50uL |
37℃C |
15min |
|
| Plasmin |
50uL |
|
90sec |
|
| Chromogenic substrate |
50uL |
|
10min |
Color development |
| Acetic acid |
50uL |
|
|
Stop reaction |
- Plate is read at 405nm at desired time points as color develops and after the reaction stop
- Absorbance are converted to %NMP alpha-2 antiplasmin using the standard curve
vonWillebrand Factor (vWF) Antigen Assay
vonWillebrand Factor antigen is detected by binding to an antibody, then secondary binding by a conjugated antibody that will produce a detectable color change with the addition of a substrate.
Additional Materials✝
- 1ug/mL rabbit anti-human vWF polyclonal antibody prepared in 50mM Na2CO3, pH 9.6
- 3% BSA in TBS
- 1% BSA in TBS
- 0.05% Tween 20 in TBS
- Horseradish peroxidase(HRP)-conjugated rabbit anti-human vWF polyclonal antibodies diluted 1/2000 in 1% BSA in TBS
- TMB peroxidase substrate
- 1N H2SO4
Procedure
- Plasma samples are diluted to 1:200 in TBS/1%BSA
- Plate wells are loaded as follows:
| Block |
100uL |
Rabbit anti-human vWF Ab |
5℃ |
Overnight |
| Block |
200uL |
3% BSA in TBS |
5℃ or 37℃ |
Overnight Or 3-5hr |
| Wash 1x |
|
1% BSA in TBS |
|
|
|
100uL |
Sample dilutions, in duplicate |
5℃ |
Overnight |
| Wash 5x |
|
TBS/Tween |
|
|
|
100uL |
HRP-conjugated rabbit anti-human vWF Ab |
5℃ |
Overnight |
| Wash 5x |
|
TBS/Tween |
|
|
| Color development |
100uL |
TMB peroxidase substrate |
|
|
| Stop reaction |
100uL |
1N H2SO4 |
|
|
- Plate is read at 655nm during development then at 450nm after reaction is stopped
- Log-log standard curve is prepared by diluting NMP 1:25 up to 1:1600 in 1% BSA in TBS, analyzed simultaneously on the sample plate
- Absorbance are converted to %NMP vWF using the standard curve
|
